EBV product overview
Based on proprietary know-how and specific reagent sets, Cyto-Barr BV has developed a number of specific tools and assay concepts for improved diagnosis of EBV in various clinical syndromes.
Serology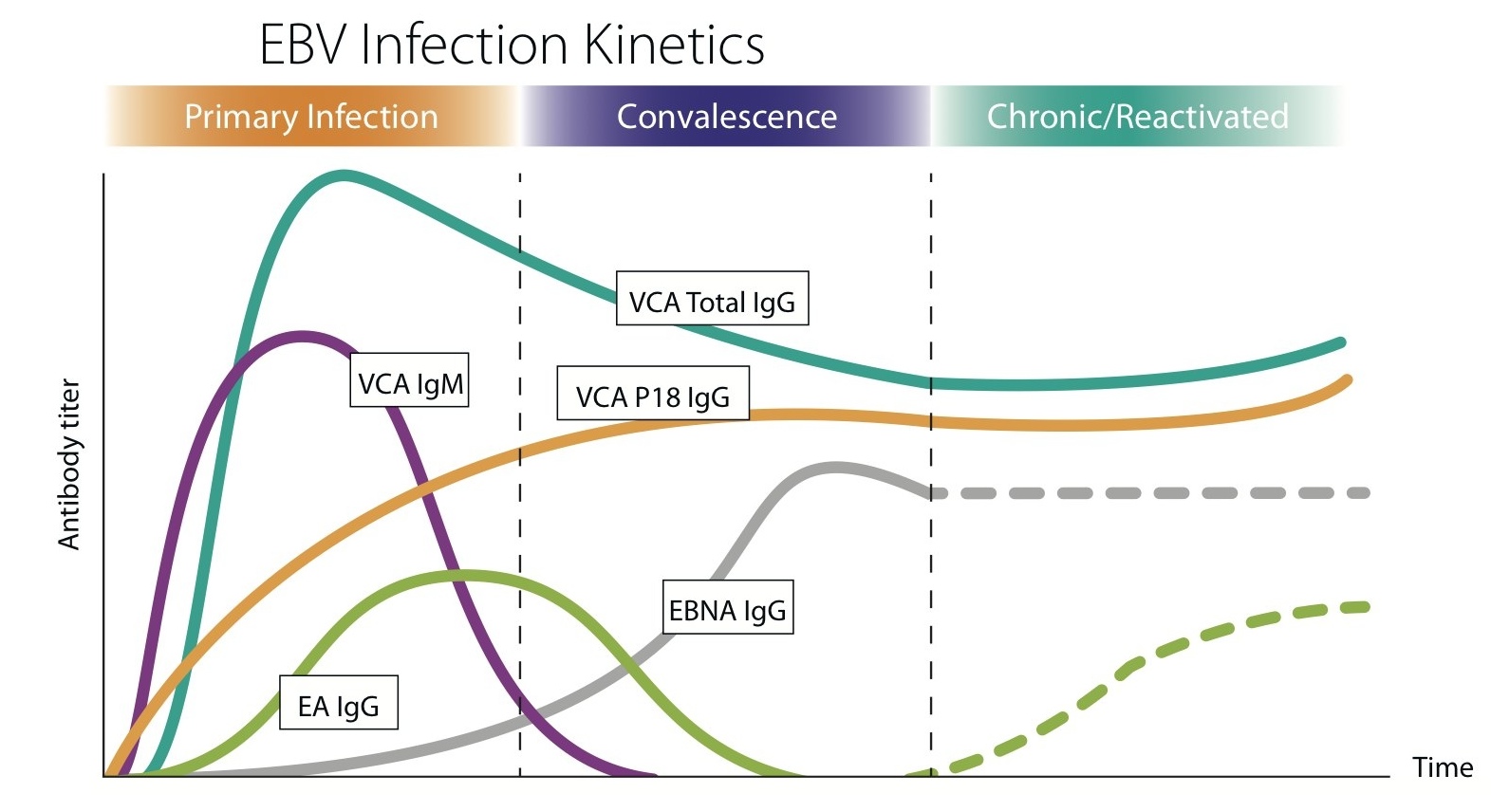
Using specific EBV-reagent combinations for:
- IM-diagnosis/-staging
- Accurate EBV carrier status definition
- CAEBV diagnosis/-monitoring
- NPC-screening/diagnosis/prognosis
- Vaccination monitoring
- EBV-immune status determination:
- IgM/IgG/IgA response to VCA, EA and EBNA
- Immunoblot profiling.
Antigen-detection
By means of a panel of Moab’s/Poab’s for in situ antigen staining for:
- Latency I-III definition
- Lytic gene expression
 DNA monitoring
DNA monitoring
By means of a competitive QT-PCR protocol
targetting a conserved region of the EBNA1 gene for:
- Monitoring EBV-DNA load in high risk patients
- Transplant recipients
- AIDS-patients and HIV-carriers
- Cancer patients
- Immunodeficiencies
- Monitoring EBV activity in CAEBV
RNA profiling
By means of EBER-RISH, (RT-)PCR or NASBA testing for:
- Demonstration of EBV presence in situ
- Latency I-III definition in biopsy specimens
